
Articles
Problems of interferences using Vapour generation Atomic Fluorescence spectrometry Mercury analysers
Posted by: chrisball | Posted on: February 8th, 2012 | 0 Comments
Vapour generation atomic fluorescence spectrometry offers many advantages in the determination of mercury. Detection limits are in the low ng l-1 range while linearity extends to ?g l-1 levels with good precision. The technique is also relatively free from interferences, which can be split into 2 classes; chemical and spectral. Chemical interferences tend to affect the vapour generation process and usually lead to a suppression of the analytical signal, while spectral interferences during the detection step generally cause a false signal enhancement. Chemical interferences can usually be overcome by selection of an alternative chemistry or by dilution of the sample, an approach made feasible by the inherent sensitivity of atomic fluorescence, while spectral interferences are minimized by the use of vapour generation as a means of sample introduction and the selectivity of the technique.

Quenching
Suppression of the analytical signal due to quenching is caused by collisions between excited atoms and diatomic gases such as O2 and N2 in the atom cell. These collisions cause a deactivation resulting in reduced fluorescence signal. The quenching can be minimized by correct choice of carrier gas to flush the analyte to the detector. Argon is the gas of choice as it has the lowest quenching coefficient. O2 or N2 can be present in samples as dissolved gases in the samples from incomplete digestion or, in the case of the determination of Hg in waters, from the addition of excessive amounts of HONH3Cl for the removal of excess oxidants. In this case Ascorbic acid can be used as an alternative pre-reductant to overcome this issue.
Spectral interferences
The nature of atomic fluorescence as a two step process of absorption of a specific wavelength followed by emission at a particular wavelength means that spectral interferences in atomic fluorescence are very rare. When coupled with vapour generation using tin(II) chloride reduction only Hg is reduced and as such gas phase spectral interference from other volatiles is unlikely especially when good digestions are used. CVAAS in comparison suffers from non specific absorption of aromatics and acid gases such as SO2 and H2S which absorb strongly at 253.7nm.
Chemical interferences
While there are relatively few chemical interferences associated with vapour or hydride generation atomic fluorescence spectrometry, there are certain species which suppress the vapour generation step. However, there are several ways to overcome such interferences, either by modifying instrumental or chemical conditions. Studies were carried out on the interferences caused in the determination of mercury by various species. The data shows that there are very few interferences, the majority of which cause suppression.
There are some interferences which should be considered. Various approaches to overcome or minimize these interferences are outlined below.
Peak shapes can be a very useful diagnostic tool. Continuous flow peaks have very characteristic shapes and if a sample peak does not have this shape it can be indicative of an interferent (although it could also be due to a pumping or gas flow problem). Figure 1 shows a typical peak shape for a mercury standard while figure 2 shows an example of a peak showing significant interference. Figure 2 is actually a peak for a sample which has undergone incomplete digestion. Immediately a peak shape like that in figure 2 is observed the analyst should recognize there is a problem. In this case the problem can be overcome simply by heating the sample digest further and diluting the sample. However, this will not always be the solution, and other approaches may be necessary.

Figure 1 Typical continuous flow peak for mercury
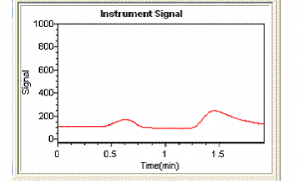
Figure 2 Hg Peak showing severe interference
Sample Dilution
As vapour generation atomic fluorescence offers such low limits of detection, many interferents can be overcome by simply diluting the sample.
Effect of sample preparation
The effect of sample preparation on Hg can be demonstrated by the following . A mercury sample containing various quantities of sodium bisulphide was prepared using HNO3 acid and also by acidification with HCl with Br-/BrO3- oxidation. The nitric acid digestion gave a low recovery because of sulphide interference whereas the bromination digestion oxidizes the sulphide and the interference is overcome by a factor of 10000.
Vapour generation atomic fluorescence for the determination of mercury and the hydride forming elements offers excellent sensitivity, linearity and is relatively free form interferences. Peak shapes are a useful diagnostic tool, allowing identification of potential interference without knowing the concentration in the sample. However, various approaches can be used on the rare occasion where interference is observed. Sample dilution, sample preparation, chemical conditions, alternative reductants or instrumental conditions can all be used to minimize any matrix effects which do occur. In addition to this, standard additions, masking agents, or separation techniques could be used.
Comments:






Leave a Comment